|
Detection of Salmonella spp. antigens in Tolima poultry products by Western Blot*
Gustavo Quintana-Ospina1, 2, 3
1 Semillero de investigación Patogénesis, Facultad de Medicina Veterinaria, Universidad del Tolima, Ibagué, Colombia. 2 Grupo de investigación Inmunobiología y Patogénesis, Facultad de Medicina Veterinaria, Universidad del Tolima, Ibagué, Colombia. 3 Grupo de investigación Avicultura, Facultad de Medicina Veterinaria, Universidad del Tolima, Ibagué, Colombia.
This email address is being protected from spambots. You need JavaScript enabled to view it.
Recibido: 02 de octubre de 2017 y Aprobado: 11 de noviembre de 2017, Actualizado: 26 de diciembre de 2017
DOI: 10.17151/vetzo.2018.12.1.6
ABSTRACT: Salmonella spp., is a Gram-negative bacterium transmitted to human by consumption of contaminated water and food consumption, mainly poultry products like eggs and chicken meat. The aim of this study was to implement the Western Blot technic to detect the presence of antigenic proteins of Salmonella spp., in chicken carcasses and egg surface and if possible to compare it with the traditional microbiological isolation. A total of 18 chicken carcasses and 18 eggs were collected from the 13 communes and 5 marketplaces of Ibagué city. A chicken carcass and egg surface washes were obtained and an aliquot from each one was processed by using standard international guidelines ISO 6579-1:2017 for bacterial isolation. Another aliquot was centrifuged and the pellet separated by polyacrylamide gel electrophoresis (SDS-PAGE). Separated proteins were transferred in to a nitrocellulose membrane by Western blot. Primary antibody was an in-house rabbit polyclonal anti-Salmonella enteritidis antiserum and the secondary antibody was a goat anti-rabbit IgG conjugated with alkaline phosphatase. The reaction of the two antibodies was detected with the addition of the BCIP-NBT enzyme substrate and the image recorded with a digital camera. Antigenic bands of Salmonella spp. of 10, 15, 17 and 40 kDa and 10, 17, 25, 37 and 75 kDa were detected in 15 out of 18 (83,3 %) and 4 out of 18 (22,2 %) samples from chicken carcasses and egg surface respectively. A total of 4 out of 36 samples were positive to Salmonella spp., by microbiological isolation. It is concluded that the SDS-PAGE and Western blot technic can successfully detect Salmonella antigens in chicken carcasses and egg surface and it may constitute a valuable complementary tool for the detection of this microorganism in poultry products.
Key Words: antibodies, bacteria, diagnosis, electrophoresis, isolation, proteins.
Detección de antígenos de Salmonella spp., en productos avícolas del Tolima, por la técnica de Western Blot
RESUMEN: Salmonella spp., es una bacteria Gram-negativa transmitida por el agua o alimentos contaminados como los productos de origen aviar. El objetivo de este estudio fue implementar la técnica de Western Blot para la detección de proteínas antigénicas de Salmonella spp., en canales de pollo y superficie de huevo y comparar dicha técnica con el cultivo microbiológico. Un total de 18 muestras de canales de pollo y 18 de huevo fueron colectadas de las 13 comunas y 5 plazas de mercado de la ciudad de Ibagué. Se obtuvo un lavado de la superficie del pollo y otro de la superficie del huevo; una alícuota fue procesada mediante el protocolo estándar internacional ISO para el aislamiento bacteriano. Otra alícuota fue centrifugada y separada mediante electroforesis en gel de poliacrilamida (SDS-PAGE). Las proteínas separadas fueron transferidas a una membrana de nitrocelulosa a través de la técnica de Western Blot. El anticuerpo primario fue un antisuero policlonal anti-Salmonella Enteritidis generado en conejo y el anticuerpo secundario, una IgG comercial de cabra anti-conejo conjugada con la enzima fosfatasa alcalina. La reacción de los dos anticuerpos fue detectada mediante la adición del substrato BCIP-NBT. Se detectaron bandas antigénicas de Salmonella spp., de 10, 15, 17 y 40 kDa y 10, 17, 25, 37 y 75 kDa en 15 de 18 (83,3 %) y 4 de 18 (22,2 %) muestras de canales de pollo y superficie de huevo respectivamente. Un total de 4 de las 36 muestras fueron positivas a Salmonella spp., por aislamiento microbiológico. Se concluye que la técnica de SDS-PAGE y Western Blot puede detectar exitosamente antígenos de Salmonella en canales de pollo y superficie de huevos y constituye una herramienta valiosa complementaria en el diagnóstico de la bacteria en productos avícolas.
Palabras clave: aislamiento microbiológico, anticuerpos, diagnóstico, electroforesis, proteínas.
Introduction
The genus Salmonella comprises 2 species: Salmonella enterica and Salmonella bongori, although a third species named Salmonella subterranea (Shelobolina et al., 2004), was recognized in 2005 and it might be incorporated in to the CDC system (Su & Chiu, 2007). Salmonella enterica is further subdivided into six subspecies that include enterica, salamae, arizonae, diarizonae, houtenae and indica and currently there are more than 2610 Salmonella serotypes (Yoshida et al., 2016), that are motile, facultative intracellular, non-lactose fermenting, Gram-negative microorganisms (Bopp et al., 2015; Quinn et al., 2001).
Salmonella is classified according to the different antigens present in the bacterial cell wall, the O antigen is known as somatic antigens and the H antigen is constituted by polymerized subunits of flagellin, whereas the virulence-associated antigen expressed in the surface of some Salmonella strains is called Vi or K antigen (Parra et al., 2002). The use of a number of commercial antisera directed to some of those surface antigens of Salmonella constitutes a universal subtyping method called Serotyping.
Several Salmonella enterica serotypes may cause infections in humans and animals; however, S. enteritidis and S. typhimurium are widely distributed and represent the main serovars associated with disease in human (Hendriksen et al., 2004; Canals et al., 2011). Salmonella is a foodborne pathogen that is usually transmitted to humans by ingestion of contaminated water and food (Velge et al., 2012), including raw chicken (WHO, 2008) and eggs (Perez et al., 2008), which are the most common sources of infection.
Foodborne illness occurs at a rate of one million, nineteen thousands hospitalizations and 380 deaths each year in the United States (CDC, 2016), meanwhile in the European Union, over 100000 human cases are reported each year (EFSA, 2017). Globally, it is estimated that 93,8 million cases of gastroenteritis and 155000 deaths are caused by Salmonella species each year (Majowicz et al., 2010), even though the number of cases is high, 60-80 % of all Salmonella infections are not recognized as part of a known outbreak and they usually are classified as sporadic cases, or are not properly diagnosed at all (WHO, 2014).
Isolation and identification of Salmonella species is a long process that requires microbiological culture and agglutination tests (Ospina-Florez et al., 2014; Van der Zee, 1994). Salmonella isolation solely is a time-consuming process that include steps like pre-enrichment, enrichment in selective agars and sub-cultivation of the samples in a variety of culture mediums (Hendriksen, 2003). To overcome those limitations, the Poultry Research Group of the University of Tolima has implemented molecular techniques to speed up the identification of Salmonella present in poultry products, among them are the use of Polymerase Chain Reaction (PCR) (Rodriguez et al., 2015), Multiplex PCR (Mogollon et al., 2016), Multilocus Sequence Typing (MLST) and Pulse Field Gel Electrophoresis (PFGE) (Rodriguez et al., 2015). The Western Blot is a fast and sensitive technic for detection and characterization of proteins based on their recognition by antibodies (de la Fuente et al., 2007). The technique has been used to detect different kinds of Salmonella proteins (Humphries et al., 2005). In this pilot study the Western Blot technique was implemented to detect the presence of antigenic proteins of Salmonella spp., in raw chicken and shell eggs, as an alternative diagnostic tool compared to the traditional microbiological isolation.
Materials and Methods
Sample collection
A total of 36 samples (18 chicken meat samples and 18 eggs) were collected from stores and supermarkets located in the 13 communes and 5 marketplaces of Ibagué city during May-November of 2016. Each sample consisted of one whole chicken leg weighing about 300 gr and 3 eggs collected at each store. The eggs were processed in a pool, thus obtaining 2 samples from each store or supermarket. The samples were packed in sterile plastic bags kept at 4 ºC and transported to the Laboratory of Veterinary Diagnosis for processing within 3 hours.
Salmonella Isolation
All samples were processed according to standard international guidelines ISO 6579-1:2017. Briefly chicken meat samples were incubated in buffered peptone water during 24 hours at 37 ºC for pre-enrichment, and subsequently an aliquot of the peptone water was seeded in Tetrathionate Broth (Muller-Kauffmann) and Rappaport Vassiliadis and incubated at 37 ºC and 42 ºC respectively for the colony selective enrichment. Later, bacterial cells were seeded on McConkey and XLT4 (Xylose Lysine Tergitol) agar. Compatible colonies were seeded in Rambach agar and confirmed as Salmonella spp., by an agglutination test with Poli AI + Vi antibodies (Difco® 222641). Positive colonies were confirmed biochemically using API® 20E gallery (Biomereux, France). The surface of eggs was rubbed in buffered peptone water and the surface wash was used as the sample and followed the same culture protocol.
Protein extraction and SDS-PAGE
To extract and separate proteins from chicken meat and egg washes, the meat pieces and pooled eggs were rinsed in peptone water for 10 minutes until a surface wash was obtained. The wash was centrifuged at 1500 rpm and the precipitated material was resuspended in PBS. A total of 50 µL of each sample was mixed with 50 µL of 2 x Laemmli buffer and boiled at 95 ºC for 5 minutes. Later samples were centrifuged at 12000 rpm at 4 ºC for 4 minutes and 20 µL of each supernatant were taken and seeded in a 15 % polyacrylamide gel. Electrophoresis was conducted at 80V for 30 minutes (prerunning) and 110V 102 minutes (running). The gel was stained with Coomasie brilliant blue overnight and discolored in decolorizing agent (water, methanol and acetic acid). Later, photographic record was taken.
Western Blotting
The proteins separated in a second polyacrylamide gel were transferred into a nitrocellulose membrane by using a Trans-Blot SD semi-dry cell (Bio-Rad) transfer equipment. The proteins from the gel were transferred at 15V for 90 minutes. After transference, the nitrocellulose membrane was blocked with BSA (TBS-Triton + BSA) for 1 hour, and washed twice for 10 minutes with TBS-T. After blocking the membrane was incubated with the primary rabbit anti-Salmonella antibody at a dilution of 1:500 for 1 hour and then washed with TBS-T twice for 10 minutes. Subsequently the membrane was incubated with goat anti-rabbit IgG conjugated with alkaline phosphatase (Sigma-Aldrich, St. Louis, MO, USA) at a dilution of 1:15000 for 1 hour. Reaction of the two antibodies was detected with the addition of 5-Bromo-4-Chloro-3-indolyl phosphate/Nitro Blue Tetrazolium (BCIP-NBT) enzyme substrate (Sigma-Aldrich, St. Louis, MO, USA).
Production of an Anti-Salmonella polyclonal antiserum
A pure culture of Salmonella Enteritidis isolated from laying hen farms (Rodriguez et al., 2015) was inactivated with 1 % buffered formalin and incubated at room temperature overnight. Inactivated bacterial cells were collected by centrifugation, washed and re-suspended on PBS. Bacterial suspension was homogenized with Complete Freund’s adjuvant (CFA) (Sigma Aldrich, St Louis, MO, USA) and inoculated into four New Zealand female rabbits via subcutaneous and intramuscular routes at different sites, with periodic booster inoculations. On day 52 post inoculation (p.i) total blood was collected from the marginal vein of the ear. The blood was left at 4 ºC overnight and, the serum was decanted and collected after centrifugation at 2500 rpm during 15 minutes at 4 ºC. Cross-reactive antibodies present in the hyperimmune serum were removed by incubation with Escherichia Coli ATCCC 25922 for 30 minutes. Bacterial cells with bound cross-reactive antibodies were removed by centrifugation at 12000 rpm and this immunoadsorption protocol was repeated twice before the serum could be used in western blot.
Results and Discussion
Western Blot analysis and molecular techniques based on immunological methods for detection of specific proteins of Salmonella spp. had been used for a long time (Cooper & Thorns, 1996; Fadl et al., 2002; Findik et al., 2010), and it has been described as an effective identification method based on potent antigenic protein detection (Maripandi & Al-Salamah, 2010). Based on those advantages of Western Blot compared to the time-consuming microbiological isolation, this study shows for the first time that this technique was effective in detecting the presence of Salmonella in poultry products in the Tolima Department.
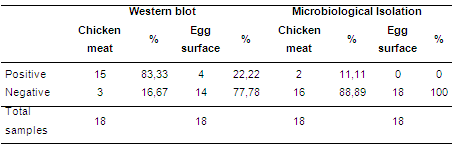
Surface washes of the chicken carcasses and eggs were processed by SDS-PAGE and Western Blot and positivity to Salmonella antigens were considered when consistent bands on the nitrocellulose membrane were apparent. A total of 15 out of 18 (83,3 %) chicken carcasses were positive for Salmonella antigens, indicating the presence of Salmonella in chicken carcasses. This result is higher than that obtained by microbiological isolation, where only 2 out of 18 (11,1 %) samples were positive for Salmonella isolation. In addition, a total 4 out of 18 (22,2 %) egg surface wash samples were found positive for Salmonella antigens even though Salmonella was not detected (0 %) by microbiological isolation of those samples (Table 1).
Table 1. Frequency of Salmonella positive poultry products by Western blotting and Microbiological isolation
The results indicate a high sensitivity of the Western Blot technique to detect Salmonella in poultry samples when compared to microbiological culture. Thus, we proposes Western Blot as an alternative diagnosis tool for epidemiologic evaluation/detection of Salmonella spp., in poultry products such as chicken meat and eggs that could speed up the identification of contaminated or Salmonella-free poultry products.
We generated a polyclonal antibody in rabbits that was immuno absorbed with E. coli cells due to presence of cross-reactive antibodies. This step seems to be very important to remove weak and non-specific antigen-antibody interactions responsible for undesirable background. Cross reactive antibodies are normally present when using polyclonal antisera and even with the use of monoclonal antibodies there could be non-specific immunoglobulins (Keller et al., 1993; Lee et al., 1989; Thorns et al., 1994). The cross-reactivity is in part due to the high similarity and amino acid identity between antigenic proteins from Enterobacteria, that could be as high as 73-82 % between E. coli, Citrobacter and Enterobacter species (Yeh et al., 2016; Nhan et al., 2011). However, to reduce the potential background caused by cross-reactive antibodies, we performed various immunoadsorption with total proteins from E. coli (Sambrook, 2001), that allowed us to detect a reduced number but specific antigenic proteins from Salmonella.
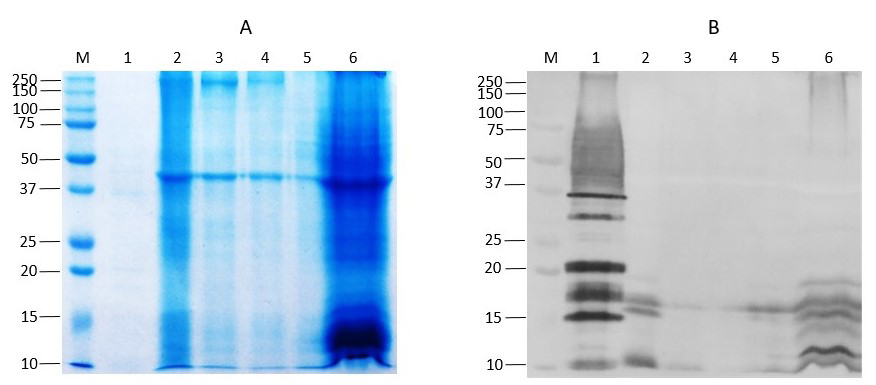
The SDS-PAGE and Western Blot assay identified antigenic protein bands of different molecular mass in both kinds of samples. In chicken carcasses protein bands of approximately 10, 15, 17, and 40 kDa were commonly detected (Figure 1B), whereas in the egg surface wash samples, bands of 10, 17, 25, 37, 75 kDa were frequently observed. Although the SDS-PAGE and Western blot assay implemented in this study could not identify the protein represented by each immunoreactive band, recent studies have reported that several Salmonella antigenic proteins corresponds to outer membrane proteins (OMPs) (Maripandi & Al-Salamah, 2010). Thus, implementing standard protocols for outer membrane proteins extraction could be useful to identify particular antigens of Salmonella with high immunoreactivity with the polyclonal IgG.
Figure 1. Detection of Salmonella spp. in poultry products by SDS-PAGE and Western Blot assay. A. Representative SDS-PAGE of total proteins from chicken carcasses and egg’s washes stained with Coomasie brilliant blue. B.Western Blot of total protein extracted from chicken carcasses and egg’s washes that shows detection of Salmonella spp., antigens M: Protein marker; Lane 1: Positive control of Salmonella enteritidis ATCC 13076; Lanes 2 to 6: Samples of chicken carcasses.
Differences in the intensity of the bands obtained in the Western Blot assay have been described previously and it is related with the genetic variability, strain, pathogenic and metabolic features of Salmonella spp. (El-Fakar & Rabie, 2009; Helmuth et al., 1985). Interestingly, the electrophoretic profile of the proteins extracted from different chicken carcasses (Figure 1A) and egg surface wash samples, produced some proteins bands visualized by SDS-PAGE that matched with antigenic protein bands detected by Western Blot, however other immunoreactive bands were not visualized by SDS-PAGE, and may suggest a different nature of those antigens, perhaps polysaccharides complexes. In addition, the amount of proteins from the positive control needed to be detected by Western Blot was significantly less than the amount of protein from chicken meat and egg wash samples. This may indicate that although the Salmonella present in each sample was significantly low it could be consistently detected by WB.
Proteins with molecular masses of 16 kDa (Koski et al., 1989), 25 kDa (Cordova, 1998) 37 and 40 kDa (Helmuth et al., 1985; Singh et al., 2007; Verdugo-Rodriguez et al., 2009), 75 kDa (Khan et al., 2003) and a fimbria of 17 kDa (Collinson et al., 1991) in different Salmonella serotypes have been identified as antigenic proteins. Future studies should use more robust techniques such as 2-dimensional gel electrophoresis (2D) to identify the antigenic proteins of Salmonella that could be explored as immunodominant antigens. In addition, recombinant DNA technology can also be used to generate recombinant proteins to assess their antigenicity and immunoreactivity with our polyclonal anti-Salmonella antibody that help us to identify the most dominant antigenic proteins.
The SDS-PAGE and Western blot assay implemented in this study showed more effectivity when compared to microbiological isolation. The Western blot assay could detect a very low number of antigenic proteins from Salmonella spp., that may indicate a low number of bacterial cells in the wash samples and they were not detected by microbiological isolation (Gonzalez et al., 2014), however the specificity of Western Blot technique was lower than microbiological isolation when the hyperimmune sera was used without immunoadsorption. Additional studies are necessary to evaluate the possibility to increase the specificity of the Western Blot technique to detect Salmonella in poultry products, for example if an antigenic protein of Salmonella is identified to have a very low amino acid identity with the proteins of other Enterobacteria, it could be possible to produce a polyclonal antiserum specifically to that antigen. This procedure could optimize the Western blot technique as an alternative diagnostic tool for Salmonella in poultry products and food and other products and eventually reduce the transmission of Salmonella to the consumer. Detection of antigenic proteins of Salmonella spp., in poultry products by Western blot may indicate the presence of live bacteria in those products but it may also possible that only bacterial cell debris were present. In this regard the low frequency of Salmonella isolation could give support to the second possibility, however, in the pursuit for high quality and safety poultry products, in the future the establishment of minimal levels of bacterial proteins in food products, as a reflect of bacterial contamination could be established and novel and more sensitive techniques developed.
Finally, the total time required for the SDS-PAGE and Western blot assay was approximately 8 hours, which was less time consuming than the standard microbiological isolation technique to detect Salmonella spp.
Conclusions
This study has implemented an additional molecular technique, the SDS-PAGE and Western Blot to detect Salmonella in poultry products in the Tolima region. The results, are promissory for the poultry field and need to be included in future projects. In addition, the study allowed the involvement of undergraduate students of the Veterinary Medicine program and constitutes an approximation to a novel work field of Veterinarians that allow them to use standard technologies and to visualize the upcoming technologies, their use in research and epidemiological investigations and academy. The regional poultry industry should pay attention to these efforts directed to understand the epidemiology of Salmonella and the safety of poultry products and the education of Veterinary students in research focused on avian health. These results without a doubt will contribute to improve the quality and hygienic conditions of poultry products before human consumption.
Acknowledgments
The authors thank the owners of shops and supermarkets that participated in the study, to Luz Clemencia Fandiño for technical assistance in Salmonella isolation and Luis Hernando Ortiz for preparing all necessary materials.
Bibliographic References
· Bopp, C.A. et al. Escherichia, Shigella, and Salmonella. En: Jorgensen, J. et al. (Eds.). Manual of Clinical Microbiology. Washington: ASMscience, 2015. p. 685-713. · Canals, R. et al. Genomics of Salmonella species. En: Wiedmann, M.; Zhang, W. (Eds.). Genomics of Foodborne Bacterial Pathogens. New York: Springer, 2011. p. 171-236. · CDC. Salmonella Homepage. Disponible en: Link. · Collinson, S.K. et al. Purification and characterization of thin, aggregative fimbriae from Salmonella enteritidis. Journal of Bacteriology, v. 173, n. 15, p. 4773-4781, 1991. · Cooper, G.L.; Thorns, C.J. Evaluation of SEF14 fimbrial dot blot and flagellar western blot tests as indicators of Salmonella enteritidis infection in chickens. Vet Rec, v. 138, n. 7, 149-153, 1996. · Cordova, C. Utilizacion de proteinas de Salmonella enteritidis como antigenos en la prueba de western blot o inmunotransferencia. Ciudad de México, México: Universidad Autónoma Metropolitana Iztapalapa. Tesis (doctorado). · De la Fuente, A.; Rodriguez, J.; Fonseca, E. Análisis de proteínas mediante electroforesis e inmunotransferencia. PIEL. Formación Continuada en Dermatología, v. 22, n. 5, p. 252-258, 2007. · EFSA. Salmonella. Disponible en: Link. · El-Fakar, S.A.Z.; Rabie, N.S. Immunogenic properties of outer membrane proteins of Salmonella in chicken. Global Veterinaria, v. 3, n. 2, p. 75-79, 2009. · Fadl, A.A.; Venkitanarayanan, K.S.; Khan, M.I. Identification of Salmonella Enteritidis outer membrane proteins expressed during attachment to human intestinal epithelial cells. Journal of Applied Microbiology, v. 92, n. 1, p. 180-186, 2002. · Findik, A.; Buyuktanir, Ö.; Yurdusev, N. LPS and Flagellin-Based Models for Serological Screening and Confirmation of Salmonella Infections in Chickens. Kafkas Universitesi Veteriner Fakultesi Dergisi, v. 16, n. 3, p. 487-492, 2010. · Gonzalez, J. et al. Microbiological Isolation of Salmonella spp. And Molecular tools for detection. Salud Uninorte, v. 30, n. 1, p. 73-94, 2014. · Helmuth, R. et al. Epidemiology of virulence-associated plasmids and outer membrane protein patterns within seven common Salmonella serotypes. Infection and Immunity, v. 48, n. 1, p. 175-182, 1985. · Hendriksen, R. A global Salmonella surveillance and laboratory support project of the World Health Organization. Disponible en: Link. · Hendriksen, S.W.M et al. Animal-to-Human Transmission of Salmonella Typhimurium DT104A Variant. Emerging Infectious Diseases, v. 10, n. 12, p. 2225-2227, 2004. · Humphries, A.; Deridder, S.; Ba, A.J. Salmonella enterica Serotype Typhimurium Fimbrial Proteins Serve as Antigens during Infection of Mice. Infection and Immunity, v. 73, n. 9, p. 5329-5338, 2005. · Keller, L.H. Monoclonal Antibody-Based Detection System for Salmonella enteritidis. Avian Diseases, v. 37, p. 501-507, 1993. · Khan, M.I.; Fadl, A.A.; Venkitanarayanan, K.S. Reducing colonization of Salmonella enteritidis in chicken by targeting outer membrane proteins. Journal of Applied Microbiology, v. 95, n. 1, p. 142-145, 2003. · Koski, P et al. Isolation, cloning, and primary structure of a cationic 16-kDa outer membrane protein of Salmonella typhimurium. Journal of Biological Chemistry, v. 264, n. 32, p. 18973-18980, 1989. · Lee, H.A. et al. Rapid enzyme‐linked immunosorbent assays for the detection of Salmonella enteritidis in eggs. Food and Agricultural Immunology, v. 1, n. 2, p- 89-99, 1989. · Majowicz, S.E. et al. The Global Burden of Nontyphoidal Salmonella Gastroenteritis. Clinical Infectious Diseases, v. 50, n. 6, p. 882-889, 2010. · Maripandi, A.; Al-Salamah, A.A. Analysis of Salmonella enteritidis outer membrane proteins and lipopolysaccharide profiles with the detection of immune dominant proteins. American Journal of Immunology, v. 6, n. 1, p. 1-6, 2010. · Mogollon, C.; Rodriguez, V.; Verjan, N. Serotyping and molecular typing of Salmonella isolated from commercial eggs at Ibague, Colombia. Revista de Salud Animal, v. 38, n. 3, p. 1-10, 2016. · Nhan, N.T. et al. Surface display of Salmonella epitopes in Escherichia coli and Staphylococcus carnosus. Microbial Cell Factories, v. 10, n. 1, p. 22, 2011. · Ospina-Florez, B. et al. Desarrollo de una prueba de Western Blot para la detección de Brucella canis en perros. Veterinaria y Zootecnia, v. 8, n. 1, p. 99-111, 2014. · Parra, M. et al. Microbiologia, patogénesis, epidemiología, clínica y diagnóstico de las infecciones producidas por Salmonella. Mvz-Córdoba, v. 7, n. 2, p. 187-200, 2002. · Perez, C. et al. Standardization of two Polymerase Chain Reaction tests for the diagnosis of Salmonella enterica subspecie enterica in eggs. Archivos de Medicina Veterinaria, v. 40, p. 235-242, 2008. · Quinn, P. Veterinary Microbiology and Microbial Disease. New York, USA: WILEY, 2001. · Rodriguez, J.; Rondón, I.; Verjan, N. Serotypes of Salmonella in Broiler Carcasses Marketed at Ibague, Colombia. Revista Brasileira de Ciência Avícola, v. 17, n. 4, p. 545-552, 2015. · Rodríguez, R. et al. Characterization of Salmonella from Commercial Egg-Laying Hen Farms in a Central Region of Colombia. Avian Diseases, v. 59, n. 1, p. 57-63, 2015. · Sambrook, J. Molecular cloning. Disponible en: Link. · Shelobolina, E.S. et al. Isolation, Characterization, and U(VI)-Reducing Potential of a Facultatively Anaerobic, Acid-Resistant Bacterium from Low-pH, Nitrate- and U(VI)-Contaminated Subsurface Sediment and Description of Salmonella subterranea sp. nov. Applied and Environmental Microbiology, v. 70, n. 5, p. 2959-2965, 2004. · Singh, R. et al. Low molecular weight proteins of outer membrane of Salmonella typhimurium are immunogenic in Salmonella induced reactive arthritis revealed by proteomics. Clinical and Experimental Immunology, v. 148, n. 3, p. 486-493, 2007. · Su, L.-H.; Chiu, C.-H. Salmonella: Clinical Importance and Evolution of Nomenclature. Chang Gung Medical Journal, v. 30, n. 3, p. 210-219, 2007. · Thorns, C.J.; McLaren, I.M.; Sojka, M.G. The use of latex particle agglutination to specifically detect Salmonella enteritidis. International Journal of Food Microbiology, v. 21, n. 1-2), p. 47-53, 1994. · Van der Zee, H. Conventional methods for the detection and isolation of Salmonella enteritidis. International Journal of Food Microbiology, v. 21, n. 1-2, p. 41-46, 1994. · Velge, P. et al. Multiplicity of Salmonella entry mechanisms, a new paradigm for Salmonella pathogenesis. MicrobiologyOpen, v. 1, n. 3, p. 243-258, 2012. · Verdugo-Rodriguez, A. et al. Detection of antibodies against Salmonella typhi outer membrane protein (OMP) preparation in typhoid fever patients. Asian Pac J Allergy Immunol, v. 11, n. 1, p. 45-52, 1993. · WHO. Foodborne diseases outbreaks: Guidelines for investigation and control. Disponible en: Link. · WHO. Salmonella (non typhoidal). Disponible en: Link. · Yeh, H. et al. Production of recombinant Salmonella fl agellar protein, FlgK, and its uses in detection of anti- Salmonella antibodies in chickens by automated capillary immunoassay. Journal of Microbiological Methods, v. 122, p. 27-32, 2016. · Yoshida, C. et al. Evaluation of molecular methods for identification of Salmonella serovars. Journal of Clinical Microbiology, v. 54, n. 8, p. 1992-1998, 2016.
* This research was funded by grants from the Central Research Office of the University of Tolima to the Semillero de Investigación Pathogenesis (Project No. 160413).
Como citar: Quintana-Ospina, G. et al. Detection of Salmonella spp. antigens in Tolima poultry products by Western Blot. Revista Veterinaria y Zootecnia, v. 12, n. 1, p. 72-83, 2018. Recuperado de: http://vetzootec.ucaldas.edu.co/index.php/component/content/article?id=246. DOI: 10.17151/vetzo.2018.12.1.6
Esta obra está bajo una Licencia de Creative Commons Reconocimiento CC BY
|